Hollister® VaPro™ Plus Pocket

Product Overview
Available Lengths: 16 inches (Male), 10 inches (Female)
Available French Sizes: Fr 8, 10, 12, 14, 16 (Male only)
Available Tips: Straight only
Pros
There’s a lot to like in this completely touchless design – the catheter will help you avoid most of the causes of UTIs.
An integrated urine bag allows you to use this catheter wherever you are, no matter what the circumstances are.
Compact design makes traveling with this catheter a breeze.
Our Verdict
This is a very solid catheter with a price tag and environmental footprint to match.
Cons
This is a pricey catheter that not everyone will receive coverage for or be able to afford.
Closed catheter systems use a lot of plastic.
There’s a vague chemical smell to this catheter.
A limited temperature range means that you need to be careful about where you store and use this catheter.
Material
The Hollister® VaPro™ Plus Pocket Catheter is made out of PVC with a PVP (polyvinylpyrollidone) hydrophilic coating, and is considered a closed-catheter system. The PVC is certified to be phthalate free, which means it does not contain DEHP, unlike a few other PVC catheters like the Coloplast® Self-Cath or the Bard® Clean-Cath®. Reports in the published literature note that use of PVC can be associated with negative health effects in humans and that its manufacture can be associated with negative environmental impacts.
More on PVP below in the lubrication section!
Flexibility
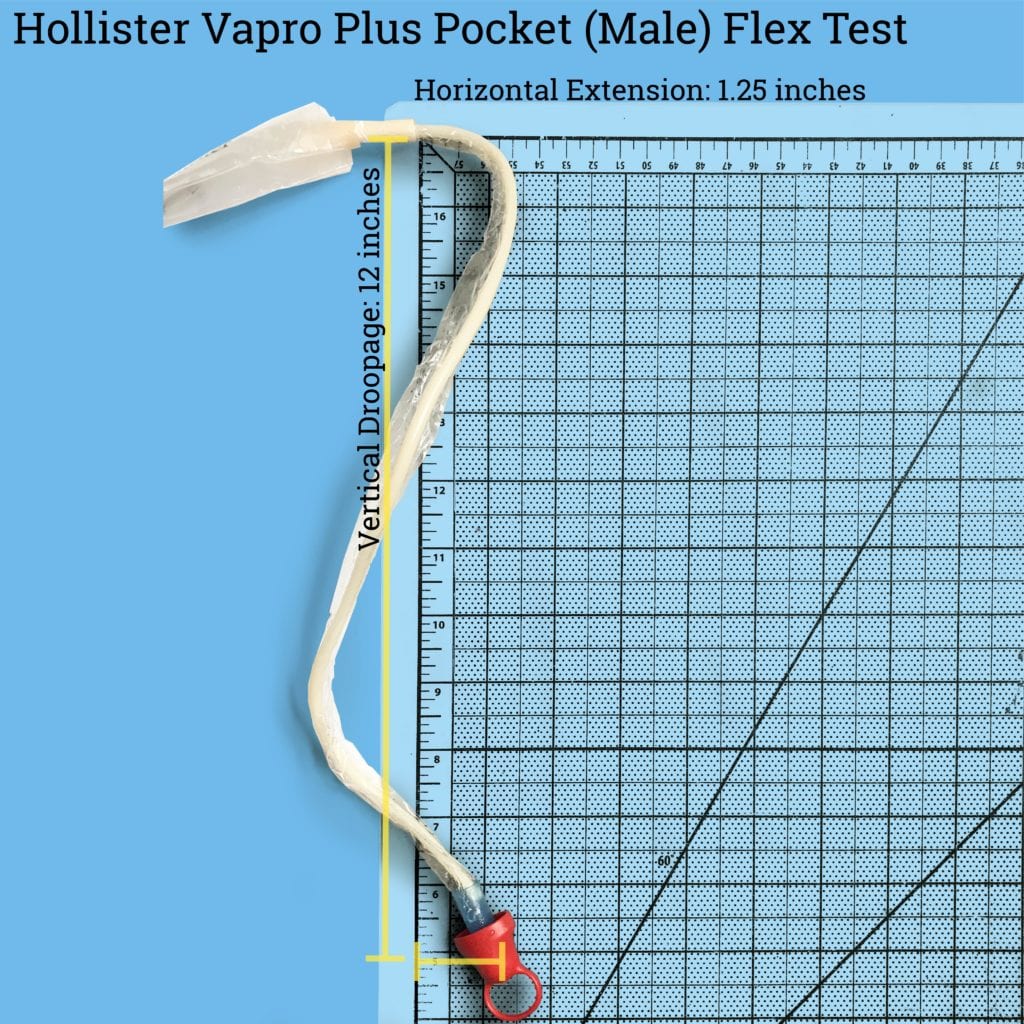
This catheter is incredibly droopy – dropping down 12 inches while extending out 1.25 inches (these are net measurements since the catheter, as you can see from the image, is pretty folded up from how it’s packaged – which we would normally consider to be a bad thing. Given that it’s packaged with an introducer tip and an insertion sleeve, you don’t run into the same issues that you would with trying to lubricate and insert a non-coated flexible catheter.
According to the FDA filing, the catheter is available with a firmer catheter, deemed the F-style VaPro™ Plus Pocket. We haven’t run into this product in the wild, but it may be helpful to know that it’s out there.
Packaging
This catheter has well considered packaging that really minimizes this catheter’s footprint, allowing you to transport it with you in your pocket. Like many other hydrophilic and more premium products, the packaging has easy-open tear holes. When you pull on the tear hole, the entire side of the packet opens up, allowing you to easily access the catheter. The attached urine bag is folded up and secured with a piece of paper tape. Because the tape is wet, it’s relatively easy to tear off.
The package itself is made out of foil, likely to prevent water vapor from escaping the packaging, which would dry out the catheter. There is an absorbent plastic-like material on one side of the packaging, likely to ensure that there isn’t an overwhelming amount of moisture in the packaging, while making sure that it is still wet. It is indeed still wet when you open it, make no mistake, and a vague, science-y, anatomy lab preservative smell pervades throughout the product.
The catheter’s introducer tip comes pre-packaged in a protective cap to make sure that it doesn’t contact any contaminants until you’re ready to insert it into the body.
The attached urine bag is designed to tear open once the catheter has been used, but doesn’t have the same level of consideration as the rest of the packaging. There are no easy-open tear holes, and the plastic doesn’t rip easily. It’s hard to imagine being able to easily tear this bag open when it’s actually full of urine without the urine getting everywhere, even if you don’t have limited dexterity. Without draining the bag, it’s possible that urine will backflow out of the catheter and leak everywhere.
For folks who care about the environment, this catheter comes with a lot of plastic.
Lubrication
PVP is the hydrophilic coating that gives this catheter its lubricious quality. PVP is generally considered to be safe, but can cause allergic reactions in rare cases – so check with your physician if you’re unsure as to whether or not this catheter is right for you.
The catheter comes pre-lubricated using water vapor, and the packaging generally does a good job of keeping the catheter most before it is opened. That being said, because the catheter doesn’t self-lubricate the way that a Bard Magic3 GO® catheter does, you’ll want to make sure you use it soon after opening it, otherwise the touchless sleeve may stick to the catheter and dry, rough residue is left on its surface. In fact, even when the catheter is hydrated, you can feel some unevenness on the surface of the catheter – we didn’t notice that on Bard or Wellspect’s products.
The packaging indicates to keep the catheter stored between 59F and 86F, which is helpful to know if you’re traveling with the catheter in your pocket (since humans are typically around 98F) or spending extended time outside of that temperature range.
The water vapor does give the entire catheter a slick and wet feel, and some users may be put off from that.
Insertion
Instructional videos for this product can be found here.
Insertion with this catheter is really a breeze. Hollister has thought of most causes of contact contamination – the insertion sleeve and introducer tip help reduce the odds of contact contamination per clinical evidence available on the Hollister website. Critically, some of the studies in this document were independently conducted (not paid for by Hollister), including one where the use of an introducer tip was specifically tied to decreased UTI incidence rate.
Make no mistake, this is a more expensive catheter, and not everyone will receive insurance coverage for it. It falls under the insurance reimbursement code A4353; catheters that fall under this category are typically recognized by Medicare as having a price of at least $8.12 per catheter. Because they’re so expensive, getting reimbursed for these types of catheters can require extensive documentation.
Adverse Events
There was just 1 complaint filed with the FDA about the VaPro™ Plus Pocket catheter in 2019. This was a complaint filed about a UTI resulting from the use of this product; the complaint mentions that the catheter was dry when it was used.

The review of FDA Medical Device Reports from 2019 provides a limited snapshot of recent product performance in the marketplace.