Bard Clean-Cath
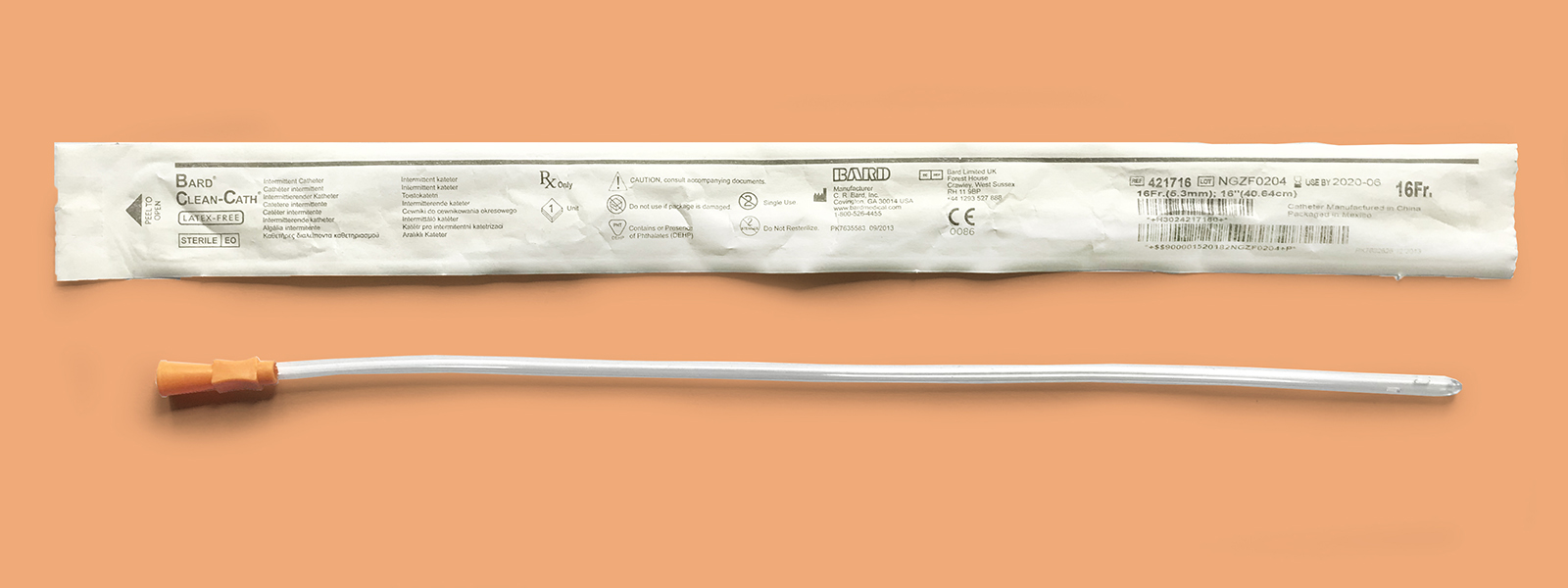
Product Overview
Available Lengths: 16 inches (Male); 10 inches (Pediatric); 6 inches (Female)
Available French Sizes: Fr 6 (Pediatric & Female only), 8, 10, 12 (Male & Female only), 14 (Male & Female only), 16 (Male & Female only), 18 (Male & Female only)
Available Tips: Coudé and Standard
Pros
The Clean-Cath is quite inexpensive, but that may come with a price.
Our Verdict
This product uses DEHP, so it’s probably best to stay away. There are other, cheap catheters that aren’t made with materials known to cause reproductive harm.
Cons
DEHP is not a good material to be putting into your body. Avoid it here as well.
Material
The Bard Clean-Cath® catheter is an uncoated catheter that comes in multiple materials. Most of the product line is made out of PVC, but there are versions of the catheter (straight-tip only) that are made out of vinyl. For the purposes of this review, we looked at the PVC Clean-Cath®, but will work on tracking down the Vinyl version as well.
It was very difficult to track down the FDA approval documentation for this product – which suggests that the Clean-Cath® brand may have been acquired by Bard at some point in its history. The packaging for the Clean-Cath® indicates that the catheter is made with a DEHP plasticizer. DEHP is known to interfere with testosterone production and is labeled as a carcinogen in California. The FDA issued a 2002 Public Health Notification advising healthcare professionals to switch to devices made out of alternative materials.

Flexibility
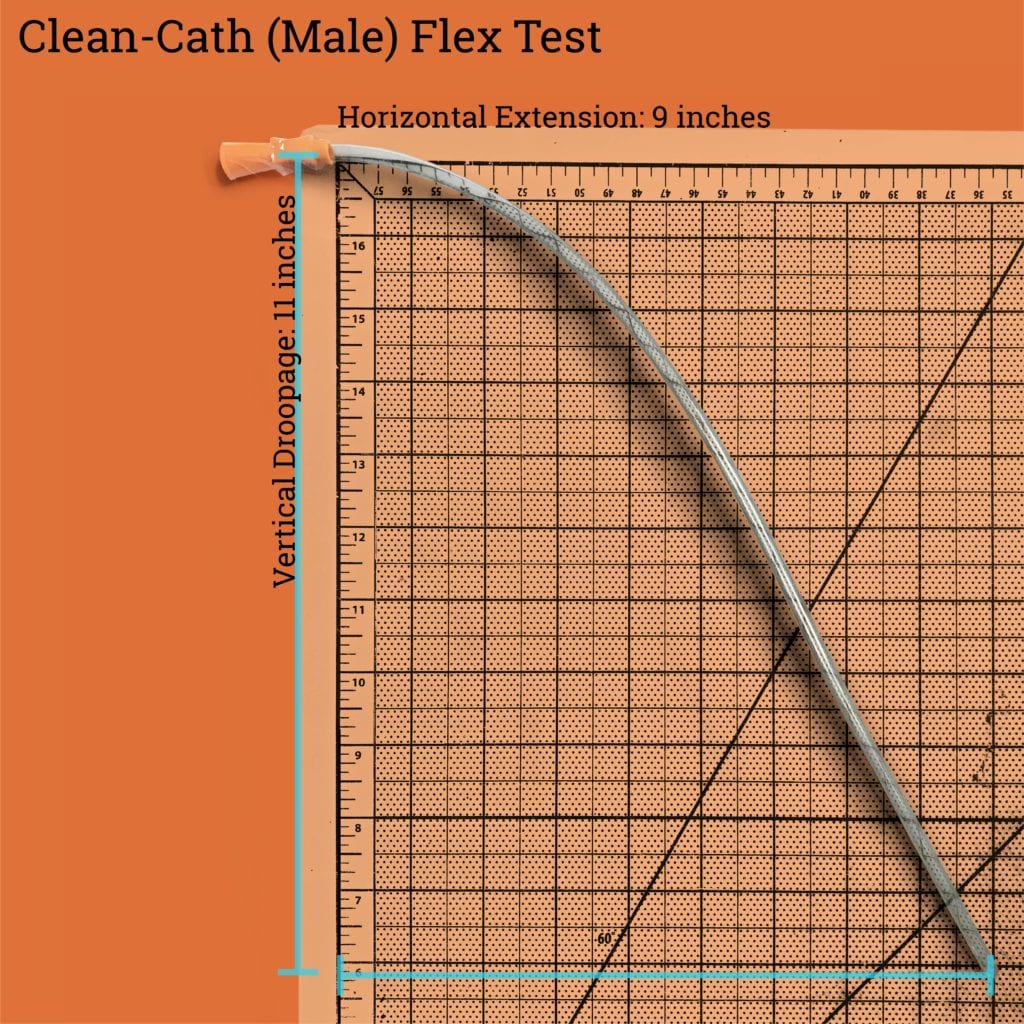 The male catheter that we tested with drooped down 11 inches while extending out 9 inches. This makes it slightly more flexible than the GentleCath Uncoated, which may present users a lot of challenges with lubrication and insertion.
The male catheter that we tested with drooped down 11 inches while extending out 9 inches. This makes it slightly more flexible than the GentleCath Uncoated, which may present users a lot of challenges with lubrication and insertion.
Packaging
The Clean-Cath® comes in very basic packaging that only opens from the funnel end of the catheter, making lubrication and handling fairly difficult. There are about 0.5 inches of real estate to grab the packaging to open it, which makes it fairly average for catheters of this type.
Lubrication
For uncoated catheters like this one, people usually take a single-use lubricant sachet, rip it open on both ends, and pass the sachet over the catheter. It’ll be hard to lubricate a catheter this flexible without getting lubricant all over your hands or without touching the entire surface of the catheter.
It is possible to use the lubricant sachet as an improvised insertion sleeve, helping you to avoid touching the catheter directly and getting lubricant all over your hands.
Insertion
If you’re a man using this product, it’s pretty much impossible to use it without directly touching every inch of the catheter as it goes into your body. The catheter does come with “finger grips” on the funnel, but with the catheter being 16 inches long, it’s hard to see what actual impact this funnel design may have on the insertion process.
We’ve known guys who remove the packaging from the catheter, and then use it to handle the catheter without touching it directly. That might be worth a shot to reduce infection potential if you think the packaging is cleaner than your hands are. Another alternative, as we mentioned above, is using the lubrication sachet as an improvised insertion sleeve.
Adverse Events
There were 58 complaints filed with the FDA about the Clean-Cath® catheter in 2019, which is fewer than Bard®’s other offerings.
There were multiple complaints of the catheter being packaged the wrong way, forcing users to touch the catheter directly when opening the package, and multiple UTI complaints. There were two complaints of the catheter tip breaking off while inside the user’s body.
To be fair, any time you’re using an uncoated catheter without an insertion sleeve or introducer tip, your UTI incidence is likely to be high unless you take several precautions like cleaning/sterilizing the urethral opening or wearing gloves
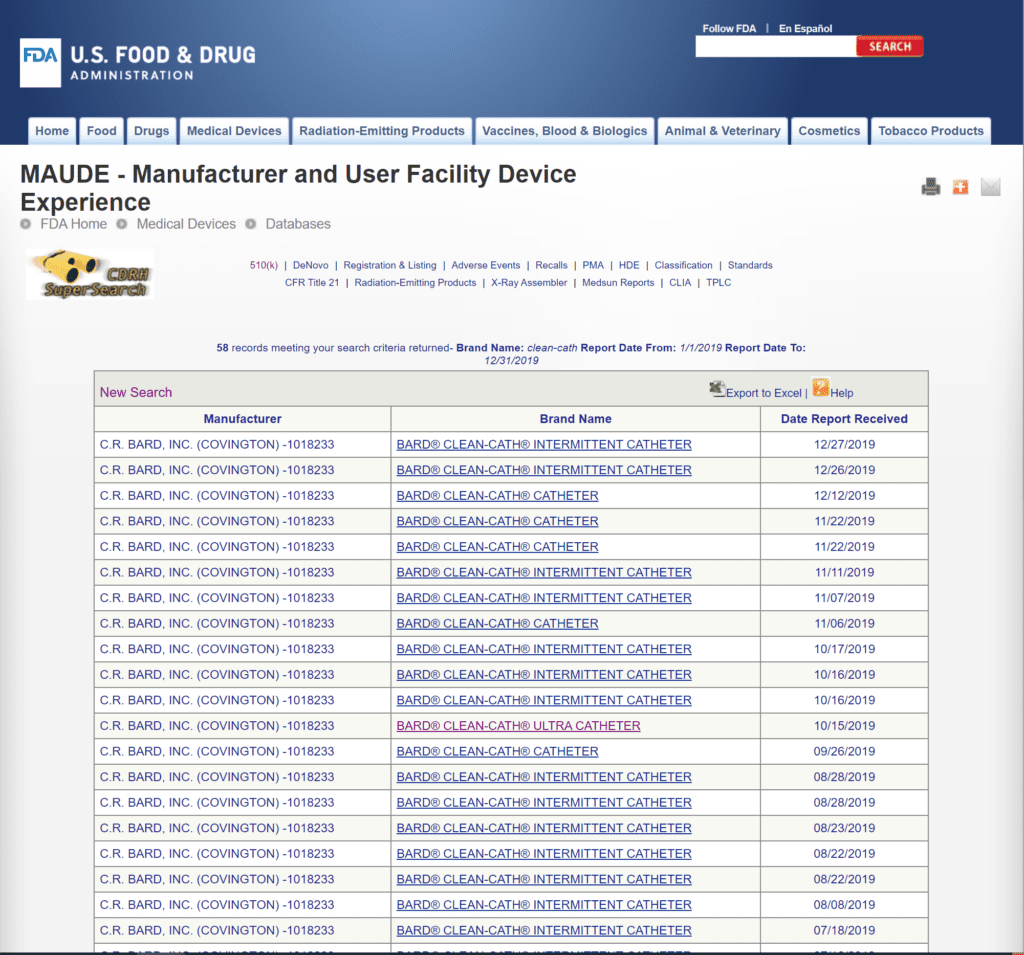
The review of FDA Medical Device Reports from 2019 provides a limited snapshot of recent product performance in the marketplace.