Convatec GentleCath™
Product Overview
Available Lengths: 16 inches (Male); 6.5 inches (Female)
Available Sizes: Fr 8, 10, 12, 14, 16, 18
Available Tips: Coudé and Standard
Pros
Extreme flexibility means you don’t have to worry about false passages.
Our Verdict
The GentleCath™ uncoated catheters are extremely flexible PVC catheters, making them difficult to handle sterilely, especially when lubricating and inserting them.
Cons
Extreme flexibility means it’s hard to avoid contact contamination.
No easy open tabs for people with limited dexterity.
Published literature suggests that there are a few issues with PVC as a material – see more in the materials section.
Material
Both the female and the male GentleCath™ uncoated catheters are made out of polyvinyl chloride (PVC) with DEHT plasticizers (which make PVC flexible and soft). Reports in the published literature note that use of PVC can be associated with negative health effects in humans and that its manufacture can be associated with negative environmental impacts.
Flexibility
Catheter flexibility is a double-edged sword – the stiffer the catheter, the easier it is to insert, but it’s also easier to accidentally injure your urethra (the linked study is specific to indwelling catheters, but the logic applies to intermittent catheters as well). On the other hand, the more flexible the catheter, the harder it is to hurt yourself while inserting it, but it’s also harder to lubricate the catheter or get it all the way to your bladder without touching every inch of it.
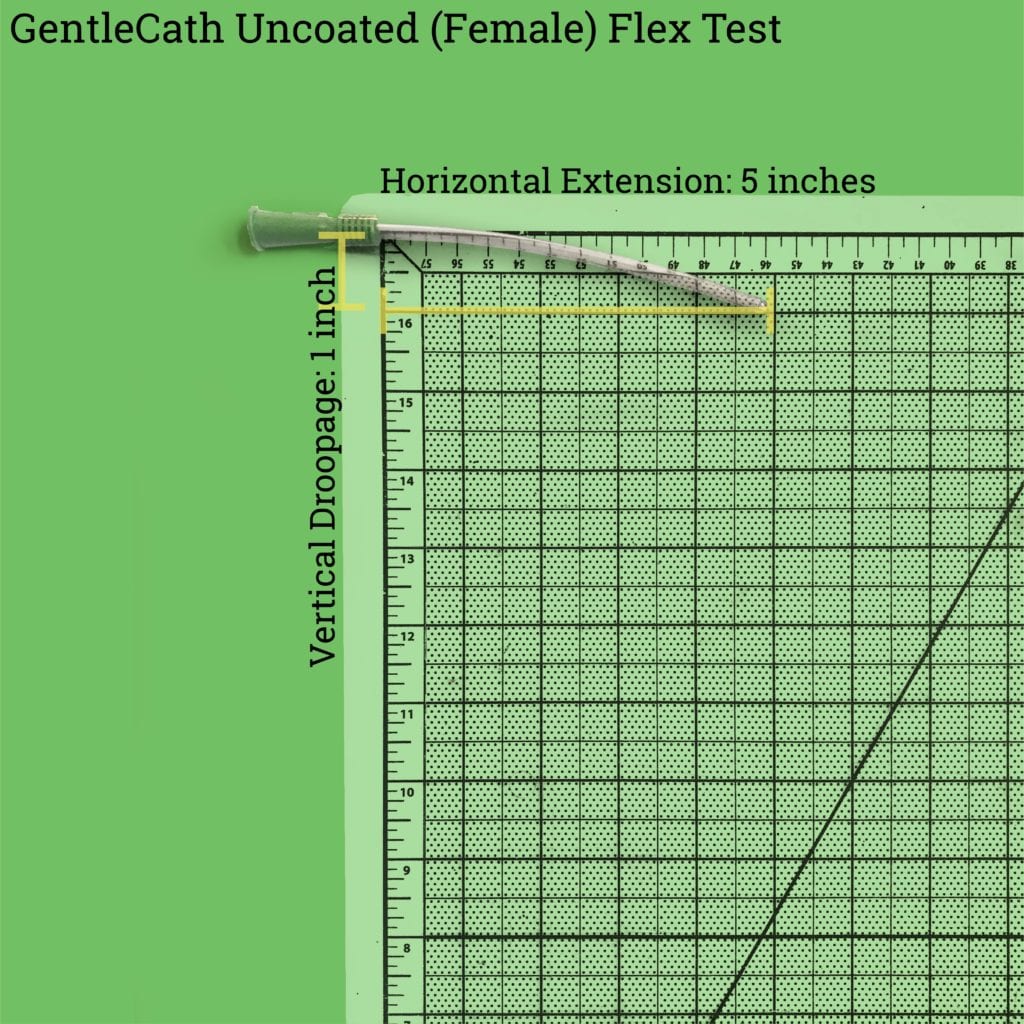
The GentleCath™ uncoated catheters are extremely flexible. The female catheter, only 6.5 inches long, droops down by about an inch when it’s held up by the funnel.
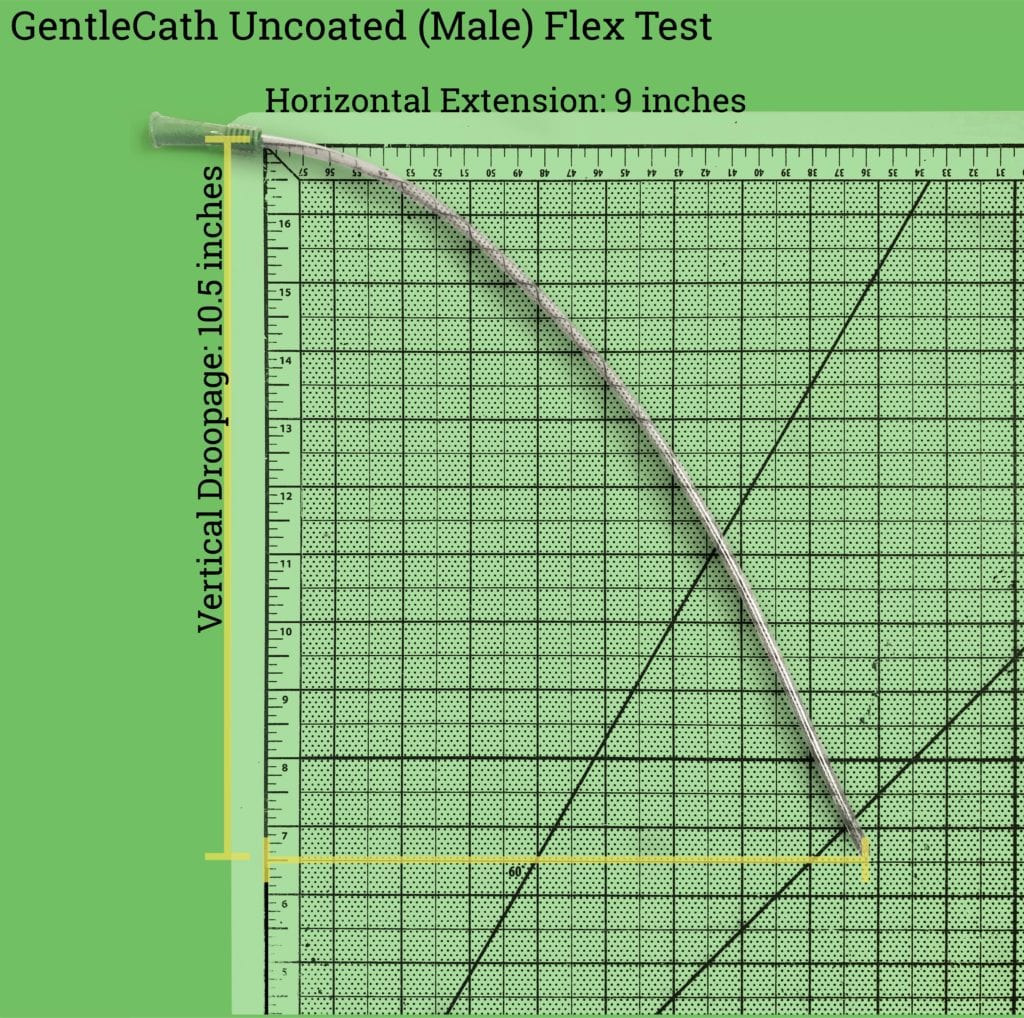
The male catheter droops down 12.25 inches, if held the right side up (where the plastic tab on the funnel is pointing up).
This extreme flexibility means that it’s really, really difficult to lubricate (see below) and insert the male catheter into the body without the catheter touching something that it’s not supposed to – which can lead to contamination of the catheter and infections. Due to its length, the female catheter is a bit easier to lubricate and insert without contamination.
Packaging
The packaging for the GentleCath™ uncoated catheter is no fuss and no frills. There are no usability tabs for people with limited dexterity, which is a drawback. There’s no sticker to temporarily place the packaging on the bathroom wall while you catheterize or any real consideration of how the catheter will be used in context.
Lubrication
For uncoated catheters like this one, people usually take a single-use lubricant sachet, rip it open on both ends, and pass the sachet over the catheter. For the female GentleCath™, this is pretty easily doable, but for the male GentleCath™, it’s a world of trouble. It’s difficult to get the catheter into the lubricant sachet, and it can be uncomfortable to handle a catheter when it’s already been lubricated (it gets all over your hands!).
It is possible to use the lubricant sachet as an improvised insertion sleeve, helping you to avoid touching the catheter directly and getting lubricant all over your hands! See our video for more details!
Insertion
The flexibility of the catheters is a major drawback here. If you’re a woman using this product, you’ll need to take care that the catheter doesn’t accidentally enter the vaginal canal when catheterizing, otherwise you’ll need to use a new catheter so you don’t get an infection.
If you’re a man using this product, it’s pretty much impossible to use it without directly touching every inch of the catheter as it goes into your body. We’ve known guys who remove the packaging from the catheter, and then use it to handle the catheter without touching it directly. That might be worth a shot to reduce infection potential if you think the packaging is cleaner than your hands are. Another alternative, as we mentioned above, is using the lubrication sachet as an improvised insertion sleeve!
Adverse Events
Two adverse events were reported in the FDA’s database for the Gentlecath™ catheters in 2019, but oddly enough the description doesn’t seem to relate to urinary catheters. Instead, it looks like the reports were for vascular catheters in both cases, and the reported manufacturer was Covidien, which is an altogether different catheter manufacturer. (Most of the complaints shown below are for the Gentlecath Glide, which is a separate product).
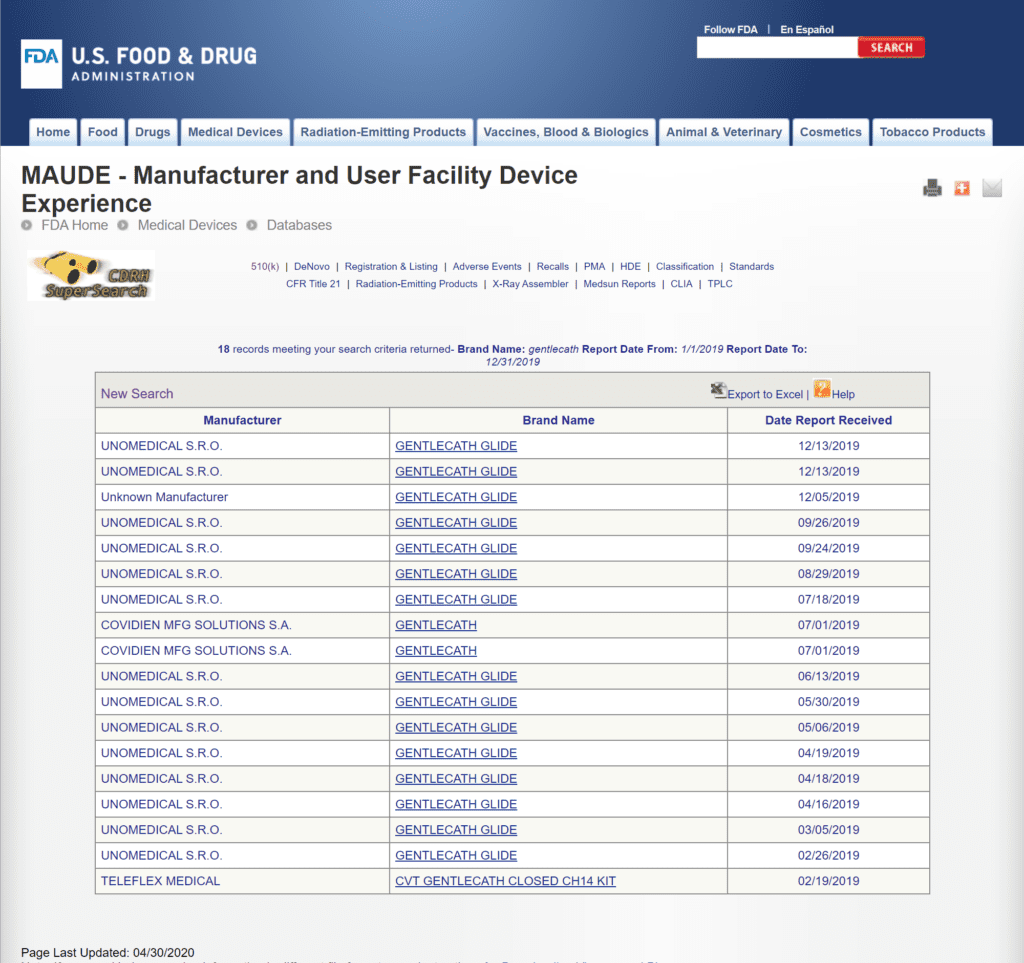
The review of FDA Medical Device Reports from 2019 provides a limited snapshot of recent product performance in the marketplace.